|
 Douglas Instruments Douglas Instruments
Research Report 2, October 1995
IMPAX 1-5
Protein Crystallization System
Harvesting Crystals from Microbatch for Cryocrystallography
Crystals were harvested to a depression plate for heavy atom derivatization
Crystals were picked up on loop straight from microbatch wells for high resolution
studies
The use of oil did not interfere with the freezing process
Research by Elena Conti, Biophysics Group, Physics Department, Imperial College,
London SW7 2BZ
Report by Patrick Shaw Stewart, Douglas Instruments Ltd.
Introduction
The microbatch method has become well established in recent years both for screening of
crystallization conditions and for the production of diffraction quality crystals (Chayen
et al., 1992). A major improvement in X-ray diffraction data collection has come with the
introduction of cryocrystallographic techniques (Petsko, 1975; Teng, 1990) which greatly
reduce radiation damage to crystals. This raises the question whether cryocrystallography
can be used with crystals grown in microbatch. Researchers are concerned that the oil,
which is used to prevent evaporation from microbatch droplets, might interfere with
harvesting crystals using a fiber loop. The different geometry of the plates used might
also impede harvesting.
In a recent project at Imperial College, crystals were successfully harvested for
cryocrystallography into a depression well containing stabilizing solution for heavy atom
derivatization. They were also harvested straight from a microbatch well for high
resolution data collection.
Production of Crystals
Crystals of recombinant firefly luciferase were initially grown by the conventional
hanging drop vapor diffusion method, but these tended to dissolve when the temperature was
changed. Heat flows from the reservoir to the coverslip in hanging drop often cause
condensation on the coverslip. For example, taking a tray out of an incubator to view
under a microscope may cause condensation. This can dilute the droplet enough to cause
crystals to dissolve. Because the luciferase crystals were found to be unstable, it was
decided to change to the microbatch method.
Crystals were grown by the microbatch method in a medium containing protein, PEG 8000,
lithium sulfate, glycerol (5%) and ethylene glycol (12.5%). The volume of the droplets for
final optimization was 4 Ál. Needles at least 1 mm long were produced with average
thickness 40 - 80 Ám. Larger crystals having thickness up to 200 Ám were occasionally
obtained but these were very fragile and tended to crack when handled.
Crystals of firefly luciferase. Data could not be collected from VD
crystals which were unstable. Courtesy of E. Conti. Acta Crystallographica. D 52 (1996),
4, pp 876-878
Screening for heavy atom derivatives was carried out using the 40 to 80 Ám crystals.
Before flash-cooling, the crystals were immersed in the stabilizing solution containing
the cryoprotective agent for a few minutes. The presence of some cryoprotectant in the
crystallization conditions prevented damage of the crystals when they were transferred
into the solution used for freezing (10% glycerol and 12.5% ethylene glycol).
Harvesting for Heavy Atom Derivatization and Structure Determination
In order to produce heavy atom derivatives it is essential to find a harvesting
solution in which the crystals would be stable. In this case, a solution containing the
same ingredients as for crystallization was used, except that the PEG concentration was 5%
higher. The following procedure was used to harvest crystals:
1. Leaving the oil in position, a whisker from a cat was used to loosen up the crystals,
and to break the crystals into sections around 400 Ám long.
2. A standard micropipette with disposable tips was used to add 15 Ál of stabilizing
solution to the well containing crystals, and the solution was gently stirred.
3. The micropipette was used to withdraw 15 Ál of solution. Usually more than one crystal
was picked up.
4. The crystals were transferred to a depression well with 100 Ál of stabilizing solution
and then to the stabilizing solution containing the desired heavy atom.
5. Cryprotectant solution was added and the crystals were mounted on rayon loops and
frozen using standard techniques.
Potential heavy atom derivatives were screened at 6 ┼ resolution using in-house sources.
A better signal to noise ratio using synchrotron radiation allowed measurement of
diffracted intensities beyond 2.7 ┼ resolution. No increase in mosaicity was observed.
Harvesting Crystals Straight from Wells
In order to increase the resolution, data was now collected from the 200
Ám crystals using synchrotron radiation. These crystals were very fragile and could not
be handled freely, so it was decided to freeze them straight from the microbatch well.
This was achieved as follows:
1. Excess oil was drained from the microbatch HLA tray by tipping.
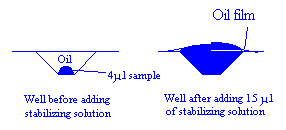
2. A micropipette was now used to add 15 Ál of cryoprotectant solution. This had the
effect of removing all but a thin film of oil from the droplet, while all the other wells
remained protected.
3. The droplet was stirred with a cat's whisker to detach the crystals, and the crystals
were broken into sections 400 Ám long.
4. The plate was inclined slightly and crystals were scooped with a loop directly out of
the droplets and frozen in the nitrogen stream.
The larger crystals and synchrotron radiation allowed data to be collected to 2 ┼
resolution.
Mounting from a microbatch well was found to be slightly more difficult than mounting from
a coverslip, mainly because of the geometry of the well. However, microbatch had other
advantages, including the exact knowledge of concentrations, and the ease with which
unmounted crystals could be transported to the synchrotron source.
References
The following papers give background information on cryocrystallography:
R. Henderson. Cryoprotection of protein crystals against radiation damage in electron and
X-ray diffraction. Proc.R.Soc.Lond. B 241, pp 6-8 (1990).
G.A. Petsko. Protein crystallography at subzero temperatures: cryoprotective motherliquors
for protein crystals. J. Mol. Biol. 96, pp 381-392 (1975).
T.Y. Teng. Mounting of crystals for macromolecular crystallography in a freestanding thin
film. J. Appl. Cryst. 23, pp 387-391 (1990).
For a description of IMPAX and the microbatch method see the following three papers:
N.E. Chayen, P.D. Shaw Stewart, D.L. Maeder, and D.M. Blow. An automated system for
micro-batch protein crystallization and screening. J. Appl. Cryst. 23, pp 297-302 (1990).
N.E. Chayen, P.D. Shaw Stewart, D.M. Blow. Microbatch crystallization under oil - a new
technique allowing many small-volume crystallization trials. J. Cryst. Growth, 122, pp
176-180 (1992).
N.E. Chayen, P.D. Shaw Stewart, P. Baldock. New developments of the IMPAX small-volume
automated crystallization system. Acta Cryst. D. 50 pp 456-458 (1994).
The following papers give examples of successful screening and optimization using
microbatch:
A. Zagari, L. Savino, S. Capasso, F. Sica, L. Mazzarella, A. Di Luccia, L. Ferrara.
Crystallization and preliminary X-ray analysis of the river buffalo (Bubalus bubalis L.)
BB phenotype carbonmomoxyhaemoglobin. Acta Cryst. (1994). D50, 778-780.
L. Pearl, B. O'Hara, R. Drew and S. Wilson. Crystal structure of AmiC: the controller of
transcription antitermination in the amidase operon of Pseudomonas aeruginosa. EMBO
Journal, vol. 13, no.24, pp.5810-5817 (1994).
Back to Research Papers Directory
|
